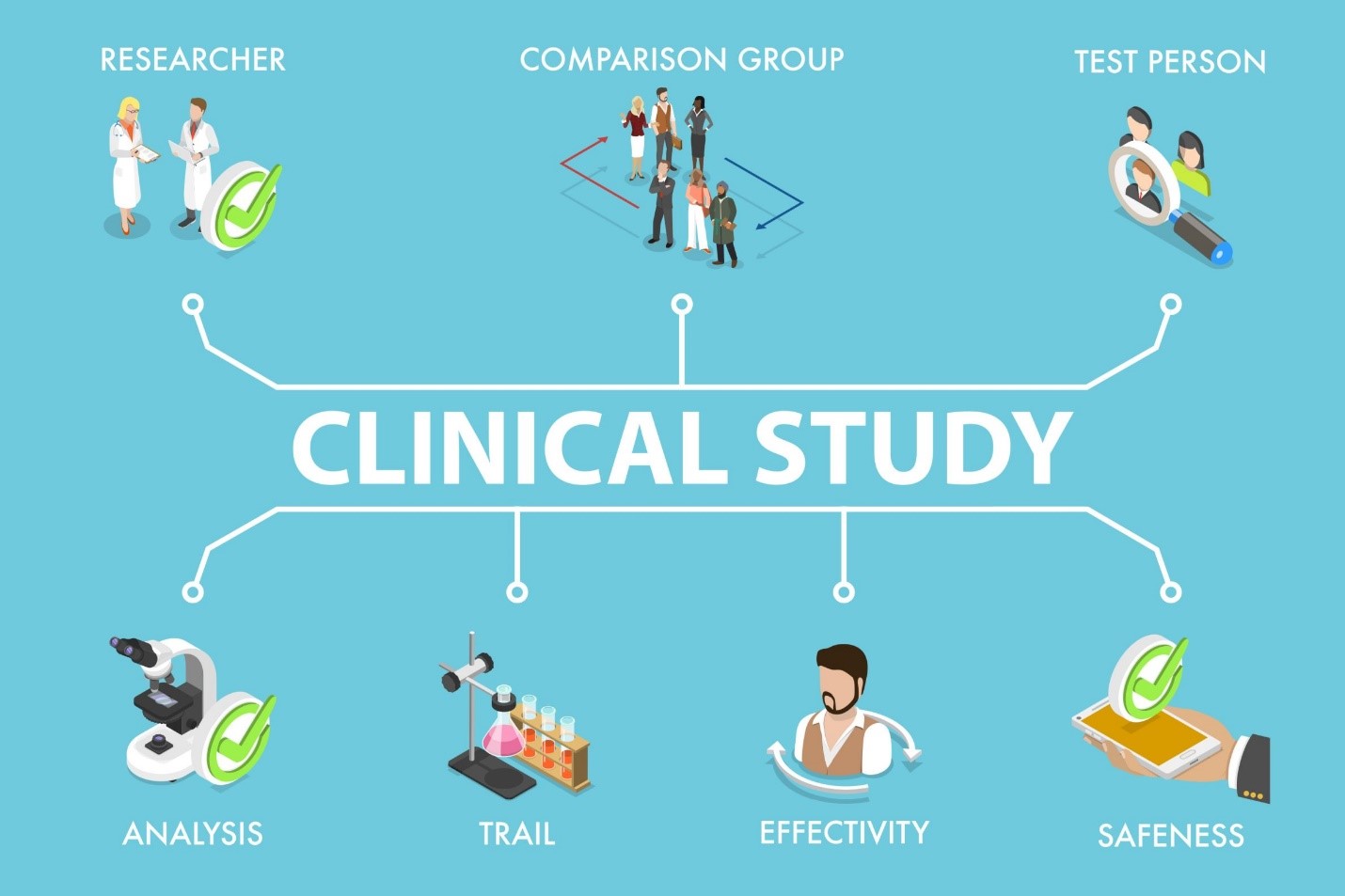
Clinical research is complex in nature. Regulatory requirements, large volumes of data, diverse teams to manage, prioritizing participants’ safety… all are daunting tasks. Most traditional processes are resource-intensive and error-prone, thus slowing down the speed and efficiency of a clinical trial.
But then came clinical research software that optimizes every stage of the clinical trial lifecycle. Everything from data collection and participant management down to oversight of trials is given a major boost. It’s revolutionizing how research is conducted. No wonder new treatments are developed at a quicker rate.
Read on to know more about how clinical study software is impacting research processes.
Enhanced Data Management and Collection
Nothing is more crucial than generating meaningful insights and drawing valid conclusions in a study design. Collecting, managing, and analyzing data has to be accurate and efficient to achieve these goals. Clinical study software provides robust electronic data capture (EDC) systems that streamline data collection from various sources, including patient questionnaires, medical records, and laboratory results.
Clinical study software solutions digitize data collection. The perks? Minimized errors. Better data integrity. Real-time access to information. Plus researchers are able to monitor trial progress, identify trends, and make data-driven decisions more confidently than ever before. EDC systems enable remote data entry as well, facilitating multi-site trials and expanding the reach of research.
The benefits of enhanced data management are substantial. EDC systems can reduce data entry errors, leading to improved data quality and more reliable study results. Real-time access to data also enables researchers to identify and address potential issues early on, potentially saving valuable time and resources.
Efficient Participant Recruitment and Engagement
Patient recruitment is a critical challenge in clinical research. Clinical study software offers innovative solutions to optimize these processes. Patient portals and mobile apps? They provide a centralized platform for communication and engagement. This means researchers can interact directly with participants, share information, and even collect data remotely.
These tools are real advocates of connection and empowerment. As a result, they enhance participant experience. Clinical research software solutions can also assist in identifying potential participants. How? They’re capable of screening electronic health records then matching them with relevant trials.
Efficient recruitment and engagement translate to faster trial completion and reduced costs. Furthermore, streamlined recruitment processes enable researchers to reach a wider and more diverse pool of participants, enhancing the generalizability of study findings.
Streamlined Trial Management and Collaboration
A team in a clinical trial process may include stakeholders such as sponsors, investigators, research coordinators, and regulatory bodies. How well the efforts of the players and their communication is coordinated may determine trial success.
Good thing clinical study software provides one place where several trial management and collaboration functions can occur. So, you can expect sharing information, study tracking, and managing documents to be a breeze. Features include shared calendars, real-time messaging, and document repositories that keep stakeholders current and aligned while promoting transparency and accountability.
The system can also automate some routine tasks, data validation and report generation included. This frees up research resources to give way to more strategic work.
Benefits for trials with streamlined management are pretty immense. Improved collaboration translates into faster decision-making, reduced administrative burden, and enhanced operational efficiency.
Robust Data Analysis and Reporting
Analysis and interpretation of complex data from clinical studies require professional tools and expertise.
Advanced statistical functions combined with visualization let the clinical study software extract meaningful insights from research and effectively communicate the findings. They can generate custom reports, build interactive charts and graphs, and even perform subgroup analyses to understand the data from many different angles. What’s more, regulatory submissions can be automatically developed by software solutions while maintaining compliance and accelerating the pace at which approval is granted.
Strong data analysis and reporting capabilities allow researchers to make evidence-based informed decisions. This paves the way for more targeted treatments.
Accelerated Regulatory Compliance and Submissions
Navigating the study protocols and regulatory requirements is another make or break aspect of clinical research. Clinical trial management software incorporates built-in compliance features, such as audit trails, electronic signatures, and automated reporting, to ensure adherence to Good Clinical Practice (GCP) guidelines and other regulatory standards.
These features streamline the documentation of case report forms, reduce the risk of errors, ensure good data security, and facilitate inspections and audits. And here’s another thing: such solutions can generate standardized reports in the required formats. The result? Accelerated regulatory submissions and increased likelihood of approval.
The benefits of enhanced regulatory compliance are significant. Streamlined documentation and reporting processes save time and resources, all while minimizing the risk of delays or rejections due to non-compliance.
Conclusion
Clinical trial software has turned into a game-changing solution in research methods. It offers precision and innovation in clinical trials through the facilitation of data management and regulatory compliance. More changes are definitely coming as technology continues to evolve, resulting in quicker development of new treatments and better patient care.
Find a Home-Based Business to Start-Up >>> Hundreds of Business Listings.